This looks pretty cool and useful for sure. It’s the size of a TV remote. It does not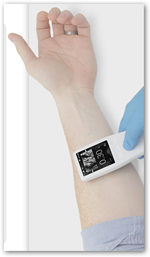 need a cart or stand to work. It does have a charging stand and has a rechargeable battery. It allows vascular access within the first ‘stick’ attempt. It is like a “window” through the skin. The company Analogic has been in the ultrasound business for years.
need a cart or stand to work. It does have a charging stand and has a rechargeable battery. It allows vascular access within the first ‘stick’ attempt. It is like a “window” through the skin. The company Analogic has been in the ultrasound business for years.
The product should start shipping this summer according to the press release. The company has a special page that tells a bit more about the product and you can sign up for updates here. BD
Peabody, Massachusetts – Analogic Corp. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the handheld Sonic Window ultrasound system, an imaging device for visualizing vasculature and guiding peripheral intravenous access (PIV). The new, ultra-compact ultrasound device provides direct visualization of structures beneath the skin in real time to effectively guide clinicians placing peripheral IVs.
"Improved visualization has been demonstrated to improve first attempt success in PIV insertion. The Sonic Window is an intuitive, handheld visualization tool that allows clinicians to quickly visualize a patient's vessel location, depth, and size," said Jim Green, president and CEO of Analogic. "Intravenous access is often one of the first needs a patient has when medical or surgical intervention is necessary. First stick success has many potential benefits: complication and cost reduction, improved time-to-care and improved patient satisfaction. With the Sonic Window acting as a 'window through the skin and into the body,' clinicians are equipped with a portable imaging device that provides clear, easy-to-interpret images on a real-time display.
http://www.onlinetmd.com/fda-analogic-handheld-medical-device-sonic-window-41214.aspx





Tidak ada komentar:
Posting Komentar